Reliable AAV Capsid Titer determination
Recombinant Adeno-Associated Virus (rAAV) vectors are increasingly used for in vivo gene transfer to target inherited genetic diseases. Yet it is essential to carry out a reliable quantification of rAAV capsid titers to ensure a safe gene transfer to the target cell.
As the industry gold standard you can rely on PROGEN’s AAV ELISAs to consistently deliver dependable capsid titer determination. You can select from a portfolio of AAV titration ELISAs and Xpress ELISAs for the most commonly used AAV serotypes. These are based on PROGEN‘s exclusive portfolio of AAV particle antibodies.

Your AAV ELISA Benefits
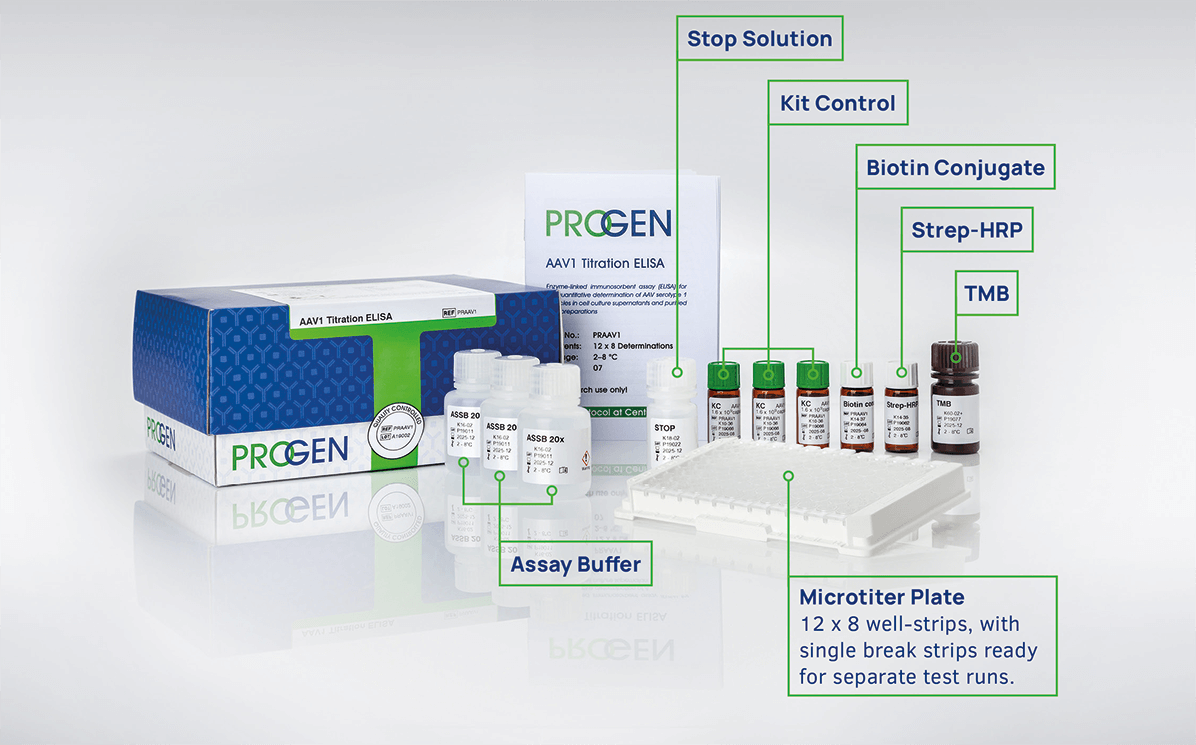
Easy-to-use: all assays use a similar workflow for all AAV serotypes and do not require expensive equipment or special training. Includes single-break strips that can be used separately for different test runs.
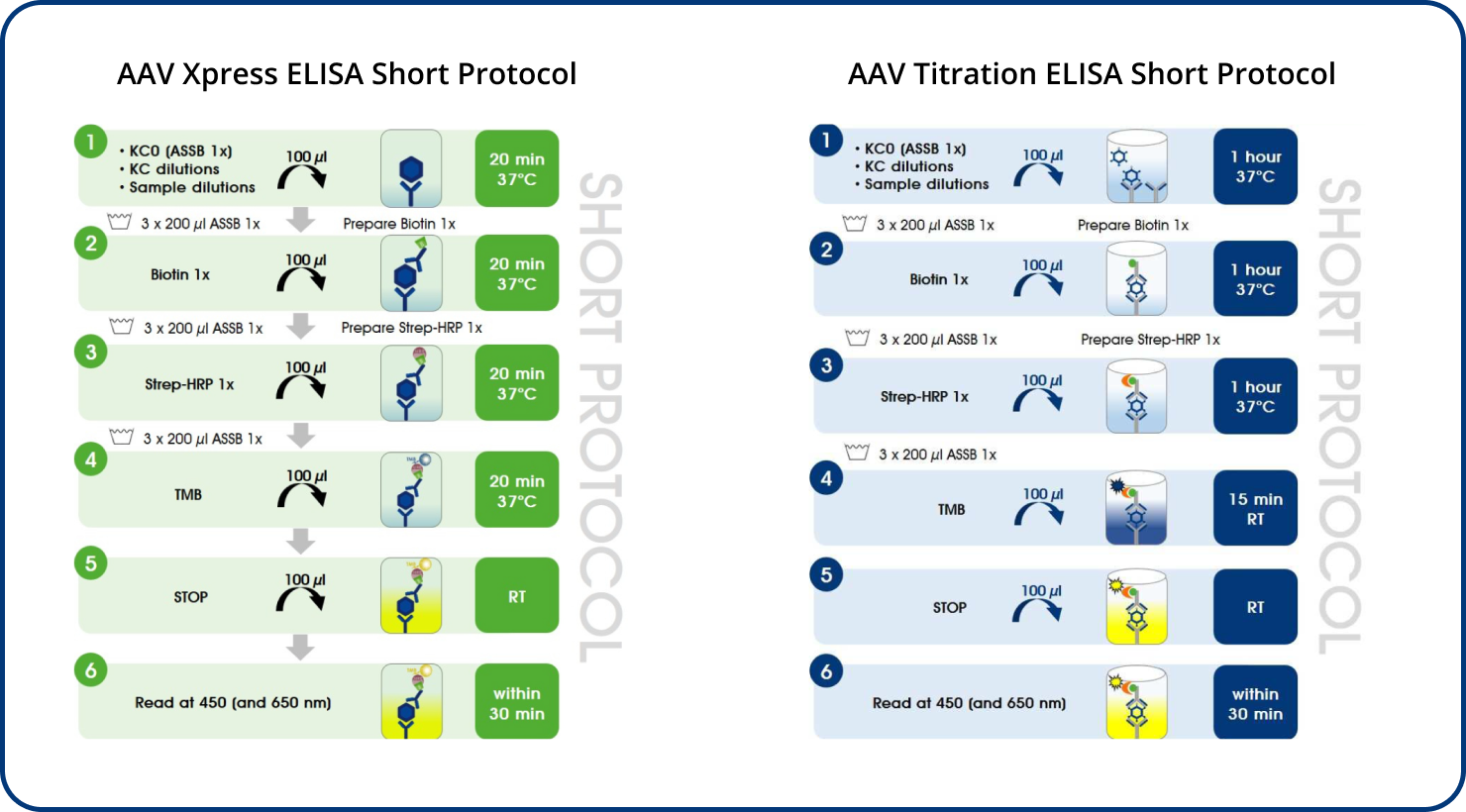
Quick: the ELISA Kits are ready to be shipped and easy to implement into all workflows. The Xpress ELISA Kits generate results in less than 2 hours without compromising performance.
Reliable: PROGEN‘s exclusive particle antibodies recognize native, non-denatured AAV capsids (full and empty) for the quantification of intact AAV particles. An industry gold standard with established success in clinical trials.
Reproducible: an established and robust method that generates reproducible data (See Figure 1). Regular AAV ELISA users can easily achieve low inter- & intra-assay variance with a coefficient of variation (CV) below 10%.
Accurate: AAV2 and AAV8 ELISAs are based on the ATCC international reference standard material and all remaining ELISAs (AAV1, 3, 5, 6, 9 and rh10) adhere to internal gold standards.
GMP-ready: can be validated for use in a GMP setting and adhere to FDA guidelines for the development of AAV gene therapies.
View All ELISAs Download Flyer

AAV Xpress ELISAs
Results in less than 2 hours
AAV Xpress ELISAs were created in response to the increasing need industry wide to get results faster and save time. The kit components have been adjusted to reduce the assay time by more than 50%. The Xpress ELISA kits have been developed based on the standard PROGEN ELISA kits, so they show the same accuracy and reproducibility. The only difference is you will be able to process a higher number of samples in less time.
Both PROGEN's AAV ELISA and AAV ELISA Xpress Kits have reference standard status in gene therapy centers worldwide.
What customers are saying
We’ve been using several kits every week and have had no trouble at all with them. We’ve been getting very good reproducibility and low variability between repeats, assays and users. Everyone is enjoying the speed of the assay and it has increased our throughput too.
Jennie Gullick, PhD, R&D Bioprocessing, Pall Corporation
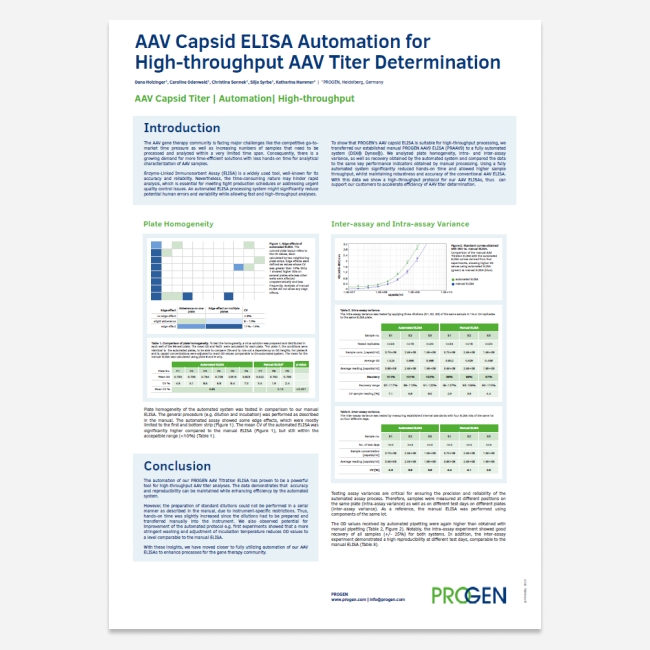
Automating the AAV ELISA
Are you Team ELISA? But looking for a solution to shift from a hands-on assay to a fully automated assay? Using the DSX® Dynex® PROGEN has made significant steps towards fully automating our AAV ELISAs.
Download our latest poster to view a high-throughput protocol for the AAV9 ELISA using the DSX® Dynex®.
Key highlights:
-
Insights on plate homogeneity, intra- and inter-assay variance as well as recovery obtained by the automated system
-
Data comparison with the manual AAV ELISA
-
Key takeaways from our automation journey
Reliable and Reproducible Data
Watch our short 90 second video and find out how you can easily implement AAV ELISAs to reliably determine the capsid titer of your AAV preparation, with no expensive equipment required.
AAV ELISA FAQs
Performance data is available here.
Yes, some of the antibodies used in our ELISA kits show cross-reactivity with other serotypes.
For more information on cross-reactivity of our antibodies, please visit our AAV particle antibody webpage.
For example, the AAV8 ELISA kits cross-react with AAVrh10. However, the AAV8 ELISA kit is not suitable for quantification of the total AAV capsid content of AAVrh10 preparations e.g. due to its serotyp-specific kit control. For quantification of AAVrh10 PROGEN offers an ELISA optimized for AAVrh10 containing AAVrh10 kit control.
With a suitable kit control an ELISA kit can be validated for a specific serotyp. For example, PROGEN offers the AAVrh74 Kit control, which can be used in combination with our AAVrh10 ELISA kit to quantify AAVrh74 capsids. This specific applications needs to be qualified by the user.
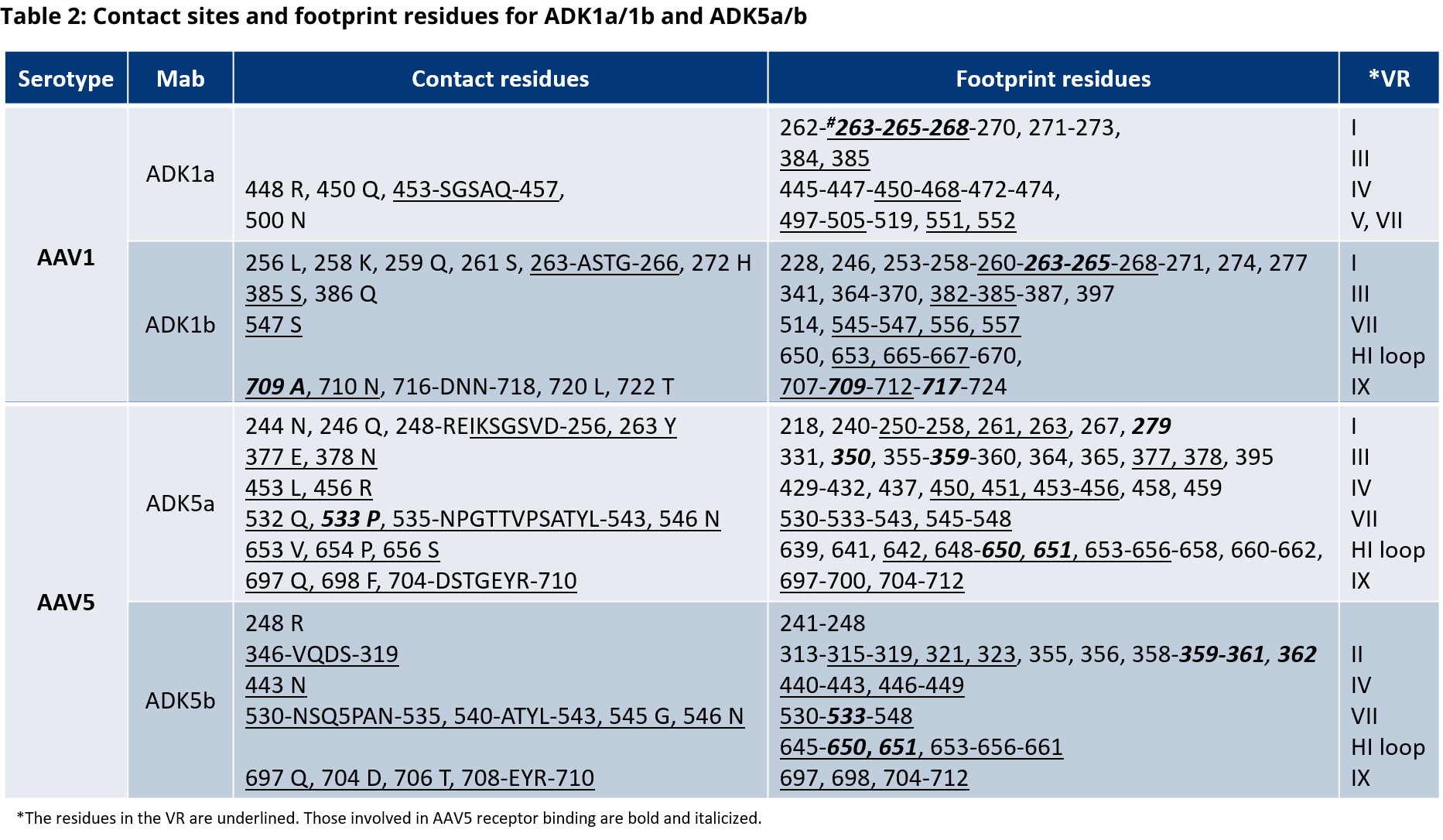
The recognition of shuffled AAV vectors depends on the specific capsid region which is affected by the shuffling. The antibodies used for PROGEN’s AAV ELISAs bind to specific conformational epitopes. These epitopes are generated by the capsid assembly of the corresponding AAV serotypes.
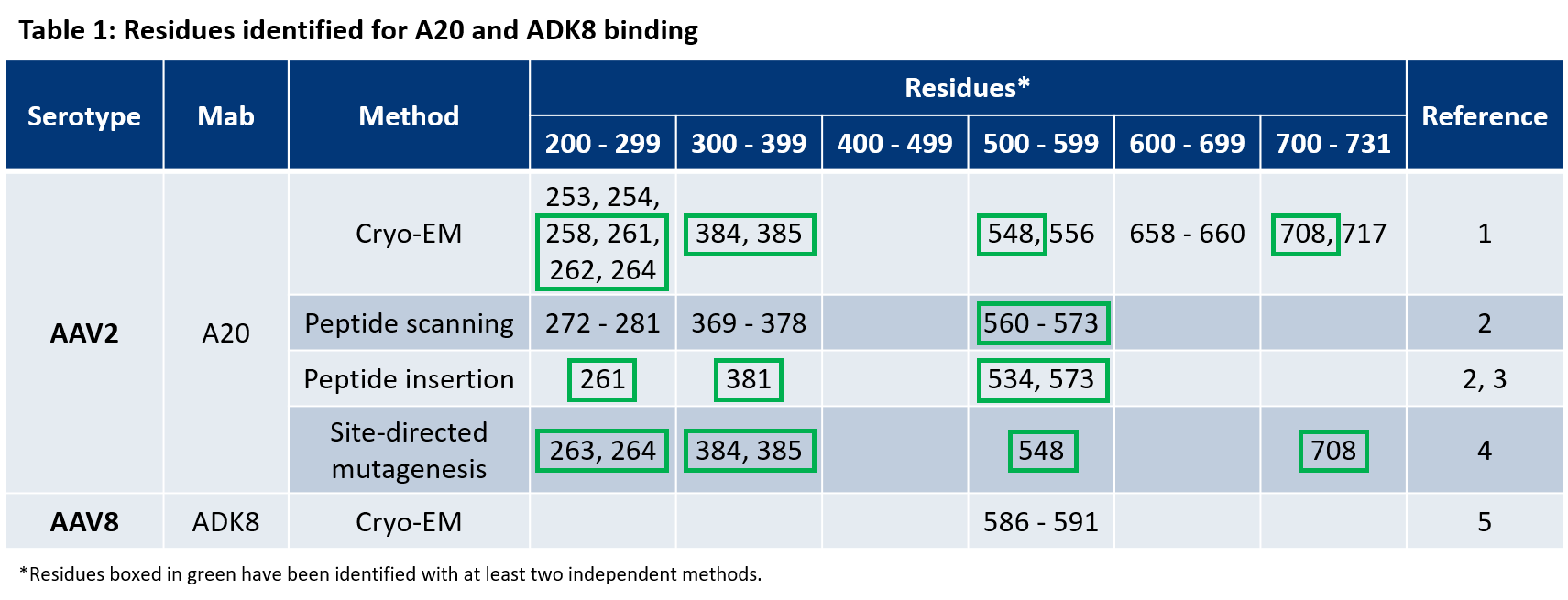

- McCraw et al. Structureof adeno-associated virus-2 in complex with neutralizing monoclonal antibody A20. Virology (2012) 431:40-9.
- Wobus, C. E. et al. Monoclonal antibodies against the adeno-associated virus type 2 (AAV-2) capsid: epitope mapping and identification of capsid domains involved in AAV-2-cell interaction and neutralization of AAV-2 infection. J. Virol. 74, 9281–93 (2000).
- Huttner et al. Genetic modifications of the adeno-associated virus type 2 capsid reduce the affinity and the neutralizing effects of human serum antibodies. Gene Ther (2003) 10:2139-47.
- Lochrie et al. Mutations on the external surfaces of adeno-associated virus type 2 capsids that affect transduction and neutralization. J Virol (2006) 80:821-34.
Yes, PROGEN offers serotype-specific AAV ELISA Controls for the standardization of your AAV titer determination.
The serotype-specific positive controls are fully characterized empty AAV capsids standardized with PROGEN´s internal gold standards* (AAV1, AAV3, AAV5, AAV6, AAV9, AAVrh10 and AAVrh74) or the ATCC international gold standard material (AAV2 & AAV8).
*For more information on PROGEN´s internal gold standard, take a look at our posters "Developing Reliable AAV Standards for ELISA" and "The New AAV3 Titration ELISA" describing the establishment and characterization of AAV5 and AAV3 internal gold standards.
Yes, for AAV particle purification we recommend OptiPrep produced by Serumwerk Bernburg AG. You can find more information on our OptiPrep site.
Our Dip'n'Check AAV lateral flow assays are perfectly suitable to estimate the approximate titer quick and easy just before performing the PROGEN ELISA. The result within 20min will guide you to the optimal dilution for your sample to hit the dynamic range. More information about our Dip’n’Check can be found on our Poster: Potential Applications for AAV Lateral Flow Tests
Bearing this in mind, once you have applied the standard curve, forty duplicate measurements can be applied.
Due to the high affinity and specificity of the AAV capture antibodies used for the PROGEN AAV ELISA kits, the detection of AAV particles from cell extracts is possible. However, detection is only possible within the reading range of the ELISA kits and reliable quantification depends on adequate titration of the corresponding samples. Furthermore, the detection of AAV capsids from cell extract can be influenced by several conditions, e.g. the composition of your lysis buffer. For example, high salt concentrations in your buffer might inhibit adequate capsid detection. We recommend to dilute the sample at least 1:10 in ASSB 1x to get a reliable result. For more information, see the following publication:
AAV Xpress ELISAs were developed based on the corresponding AAV Titration ELISAs.
PROGEN offers AAV Titration ELISA kits for the quantification of AAV serotypes 1, 2, 3, 5, 6, 8, 9, and rh10 and AAV Xpress ELISA kits for AAV serotypes 2, 5, 6, 8, and 9. AAVrh74 capsids can be quantified with a combination of AAVrh10 ELISA and AAVrh74 kit control as standard, since the antibody used in the AAVrh10 ELISA also recognizes AAVrh74.

AAV Titration ELISA
ELISAs for quantification and detection of full and empty capsids.
AAV ELISA Controls
Reliable positive controls in detections and quantification assays.