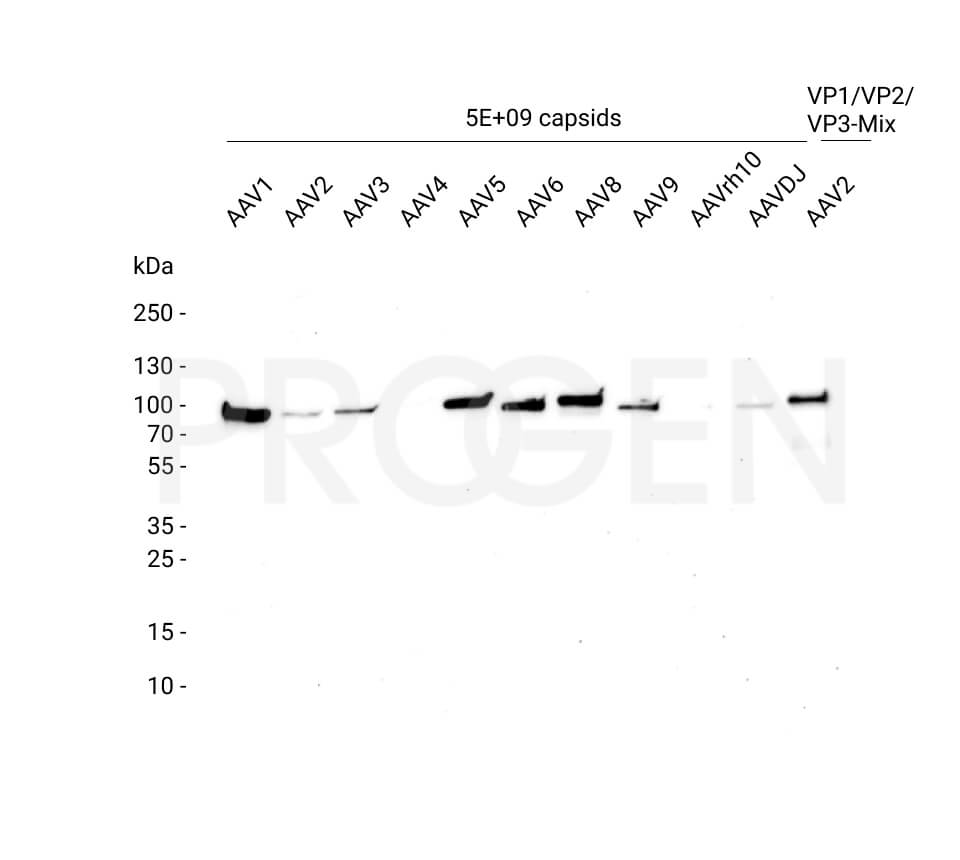
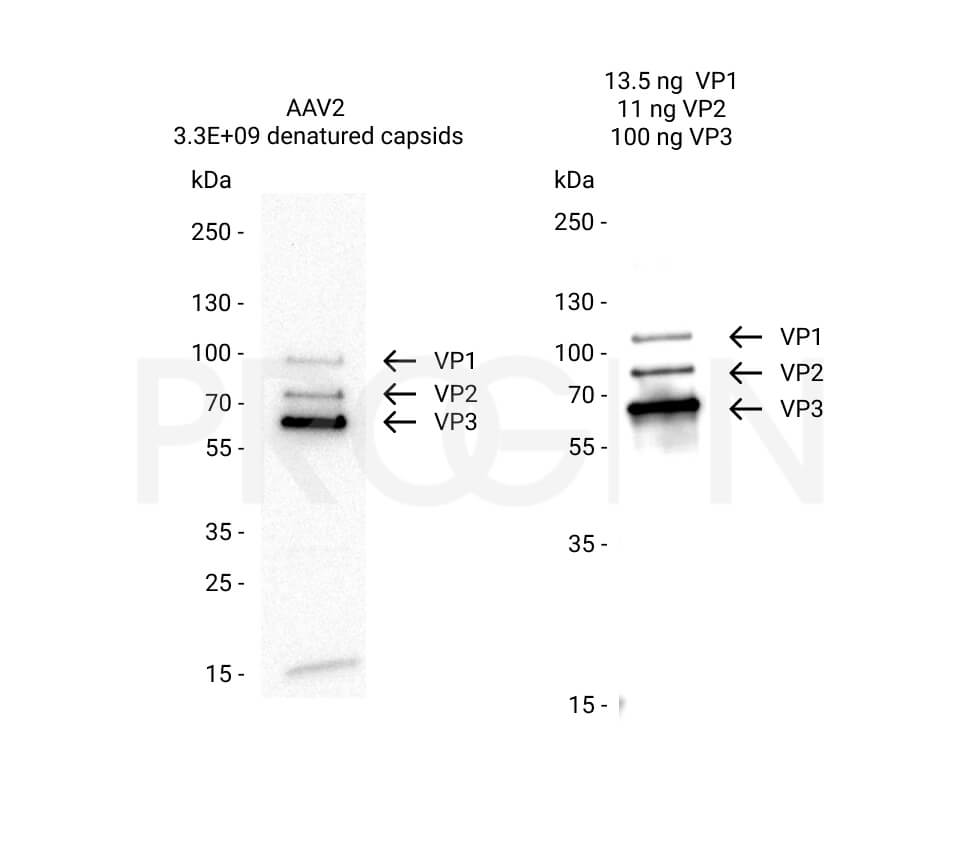
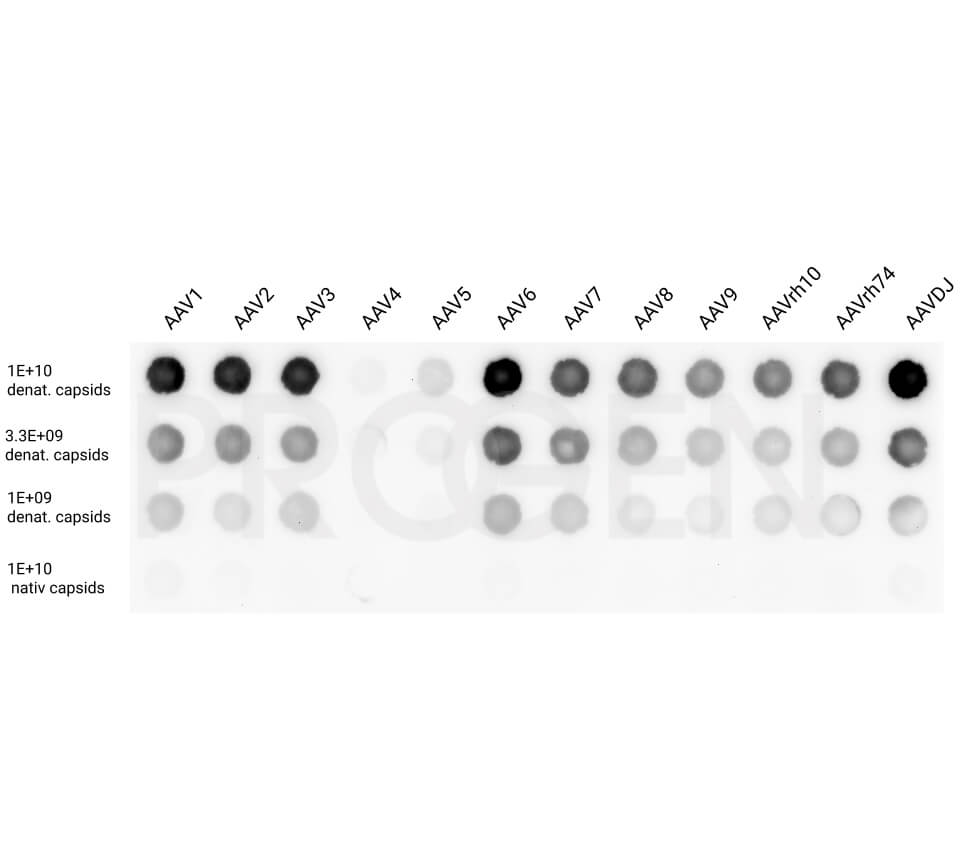
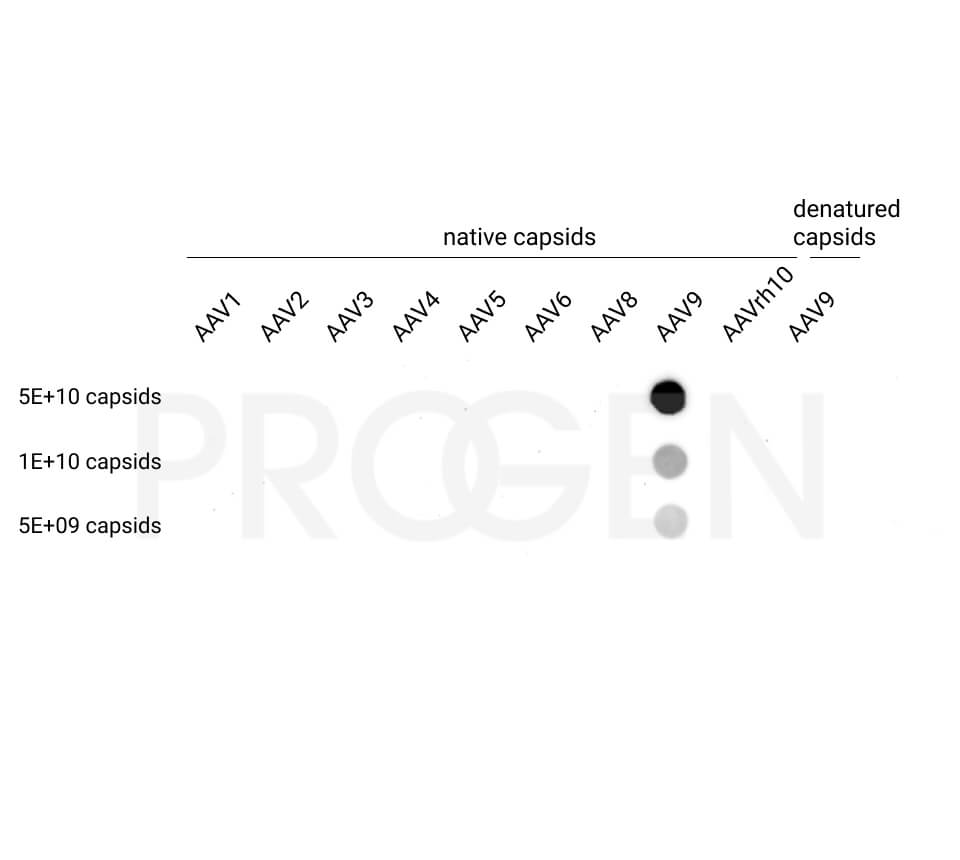
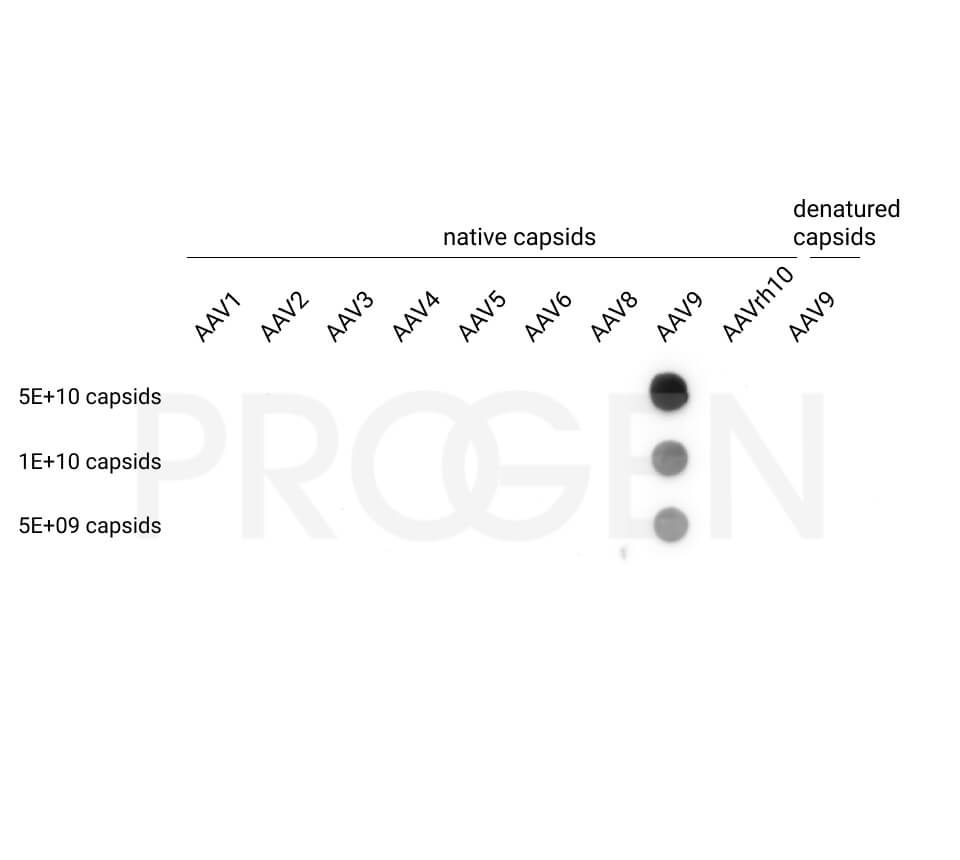
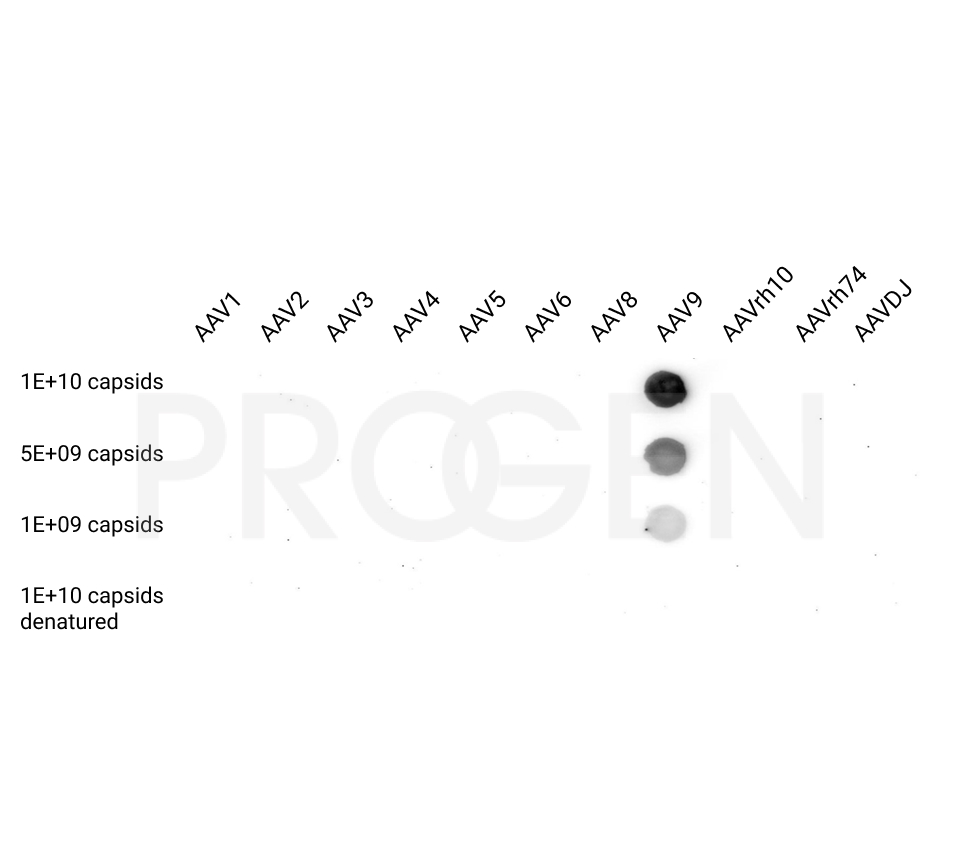
AAV9 empty capsids
- Fully assembled, empty AAV9 capsids
- Serotype-specific conformational epitopes
- Reliable positive control for dot blot, western blot, ELISA
- Quality controlled for purity, AAV titer and filling grade
- Alignment to internal reference standard material
For US shipment, the packages are only sent out for delivery on Mondays and Tuesdays, in the EU from Monday until Wednesday.
Product description
| Quantity | 100 µl (> 5.0E+11 capsids) |
|---|---|
| Application | Dot blot, ELISA, WB |
| Purification | Affinity Chromatography (POROS CaptureSelect AAV9 Affinity Resin, Thermo Fisher Scientific) |
| Storage | Up to 2 weeks: 2-8°C; long term storage in aliquots at -80°C; avoid > 5 freeze/thaw cycles |
| Intended use | Research use only |
| Concentration | > 5.0E+12 capsids/ml; please find the lot-specific concentration on the CoA and on the vial |
| Formulation | PBS + 0.014% Tween20 + 1 mM MgCl2 + 2.5 mM KCl |
| Source | Produced in HEK293T cells |
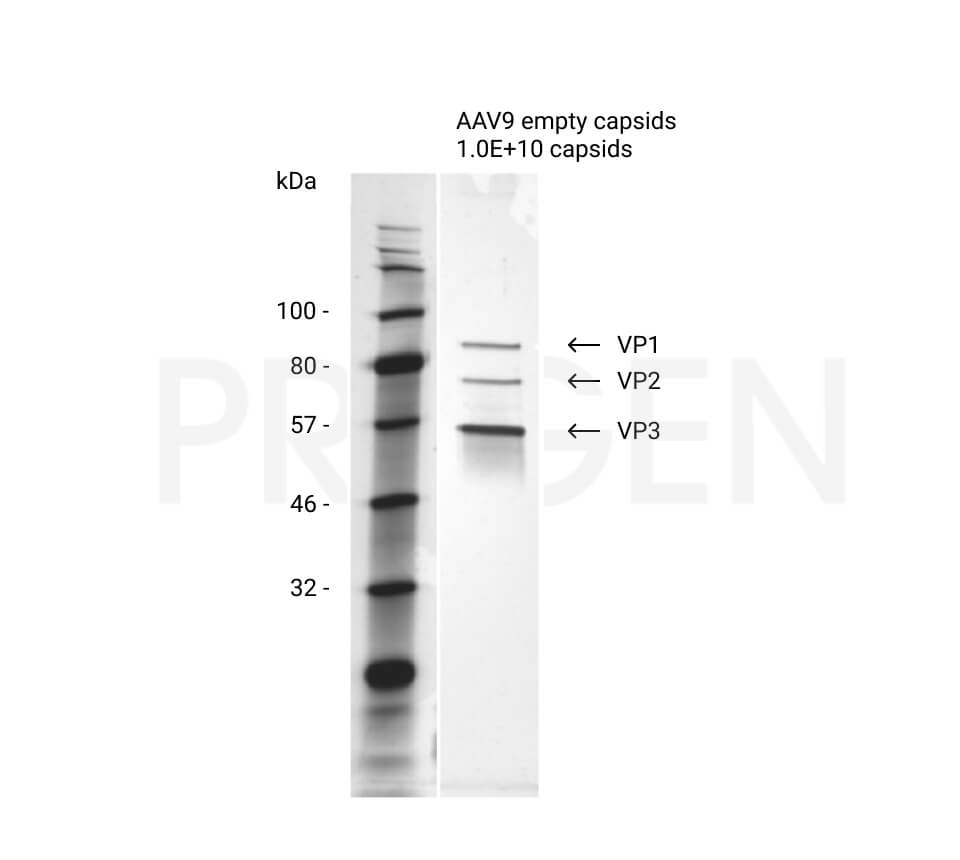
| Purity | > 95% by SDS-PAGE and silver stain |
| Quality check | Final titer was assigned on internal reference material using two AAV9 ELISA kits (PRAAV9 and PRAAV9XP); QC included analysis of filling grade and Endotoxin testing |
| Packaging Plasmid | pRep2-Cap9 + pHelper |
| Product description | Purified empty adeno-associated virus 9 capsids (AAV9) |
| Endotoxin | < 1.0 EU/ml |
| Note | Please centrifuge before opening to ensure complete recovery of vial content; repeated freeze/thaw cycles and aliquoting can lead to a drop in titer |
Applications
| Tested applications | Tested dilutions |
|---|---|
| Dot Blot | Depending on primary antibody and detection method |
| ELISA | As a positive control in ELISA, dilute in ASSB 1x (provided with PROGEN’s AAV9 ELISA) and analysis at least in duplicates is recommended |
| Western Blot (WB) | Depending on primary antibody and detection method |
Background
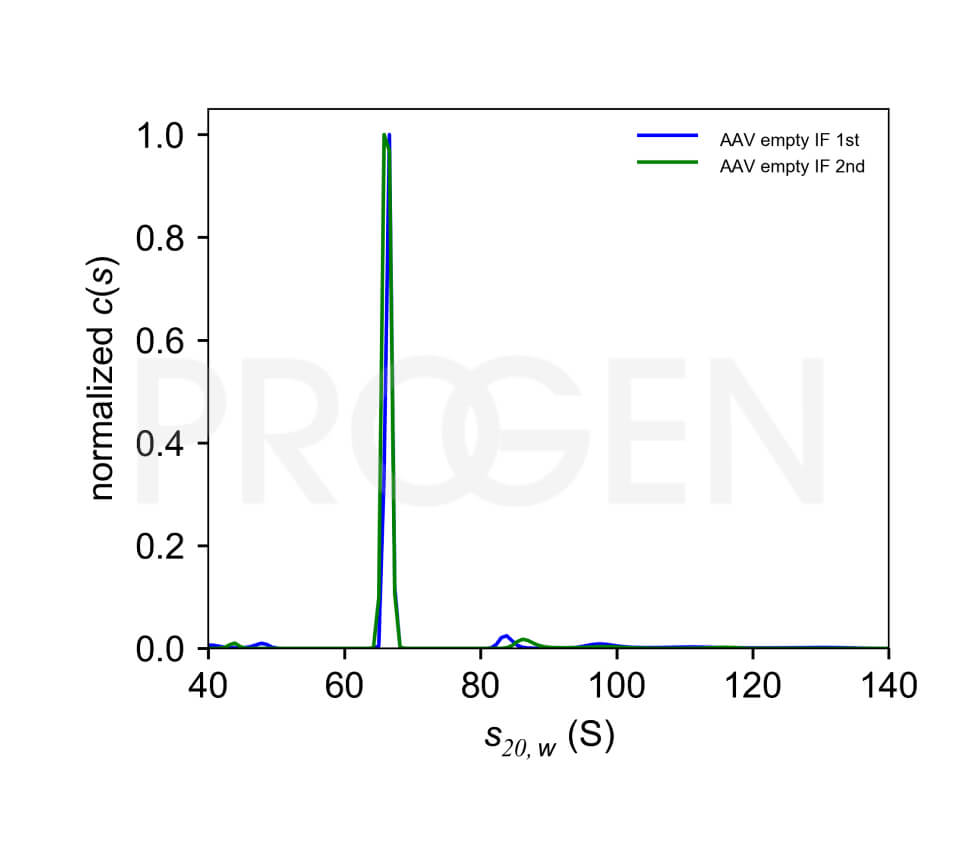
The AAV9 empty capsids are provided with titers above 5.0E+12 capsids/ml in a liquid formulation. Since the buffer does not contain any stabilizing proteins or dyes, the capsids can be used in various applications, including dot blot, western blot and ELISA. The lot-specific titers were assigned according to our internally established standard material* using two different AAV9 ELISA (PRAAV9 and PRAAV9XP).
Our comprehensive quality control ensures well-characterized capsid material which can be implemented as reference material in a variety of assays to prove the validity of the corresponding assay.
PROGEN also provides empty capsid material for the AAV serotypes 1, 2, 5, 6 and 8.
*Our internal standard material for each serotype was characterized according to the protocol described in our poster „Developing Reliable AAV Standards for ELISA“ (available in the downloads tab). Data on the establishment of standard material for specific serotypes can be found as part of the performance data for the corresponding ELISAs or can be provided upon request.
Downloads
Q & A's
Customer Reviews
Login
FAQs
The empty capsids are prepared in the absence of an ITR plasmid containing the AAV vector genome, so that no viral vector DNA can be packaged in the particles. In some rare cases the preparations may contain a very small amount of capsids in which fragments of the host cell genome and the packaging plasmids or other DNA fragments have been packaged.
Empty capsids (AAV1, 2, 5, 6, 8, 9, rh10 and rh74) are provided in liquid format in PBS + 0.014% Tween20 + 1 mM MgCl2 + 2.5 mM KCl without protein additives. The titer is greater than 5.0E+12 capsids/ml. Therefore, they can be used in several applications.
ELISA Kit Controls (AAV1, 2, 3, 5, 6, 8, 9, rh10 and rh74) are lyophilized. After reconstitution in ASSB 1x the final solution contains stabilizing protein, phenol red and ASSB 1x buffer. The titer is lot-specific around 7.9E+09-1.3E+10 capsids/ml. This product is mainly suitable for the ELISA.
The total capsid titer of our empty capsids is determined by PROGEN ELISA.
Analysis of the filling grade is performed by AUC or an orthogonal method such as UV/Vis.