- Ready-made, sterile and endotoxin tested solution of sodium diatrizoate and polysaccharide
- Suitable for the isolation of human polymorphonuclear leucocytes (granulocytes) from whole undiluted blood
Product description
| Quantity | 1x250 ml |
|---|---|
| Intended use | Research use only |
| Product description | Polymorphprep™ is a ready-made, sterile and endotoxin tested solution for the isolation of polymorphonuclear leukocytes (PMNs) from whole undiluted human blood. The solution contains Sodium Diatrizoate and a Polysaccharide |
| Density | 1.113 +/- 0.001 g/ml |
| Composition | Sodium Diatrizoate 13.8% (w/v), Polysaccharide 8.0% (w/v) |
| Osmolality | 440-500 mOsmol/kg |
| Endotoxin | < 1.0 EU/ml |
| Stability & storage | Polymorphprep™ is stable for 3 years provided the solution is kept sterile and protected from direct sunlight. Prolonged exposure to direct sunlight leads to release of iodine from the sodium diatrizoate molecule. This effect is negligible when working with this solution on a day to day basis. Polymorphprep™ should be stored at room temperature (+4°C to +30°C). |
Background
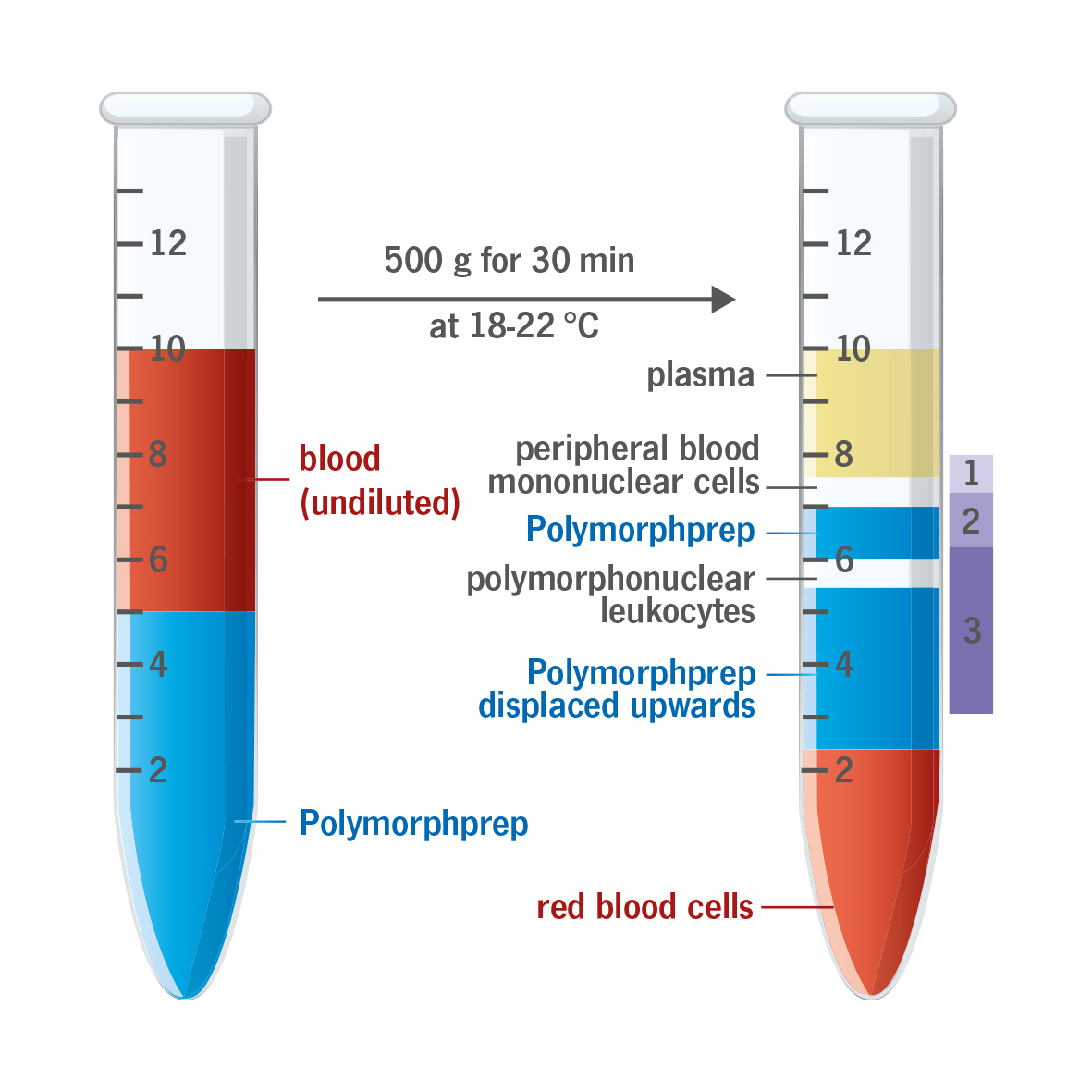
With the exception of the basophils, polymorphonuclear leukocytes (PMNs) have a much greater buoyant density than the mononuclear cells, >1.085 g/ml. Unfortunately, the buoyant density of the erythrocytes tends to be from 1.09 - 1.11 g/ml, this makes a separation from whole blood using a density barrier similar to that used for mononuclear cells more difficult. A number of procedures have been developed in an effort to overcome these difficulties.
The high osmolality of PolymorphprepTM causes erythrocytes to lose water and shrink, thus increasing their effective buoyant densities. This allows the dextran aggregated erythrocytes to sediment rapidly through the dense medium.
Because the osmotic gradient between the medium and the erythrocytes declines as the cells sediment further into the medium (ie the water loss from the erythrocytes is greatest at the top of the PolymorphprepTM and progressively decreases as they sediment further) - a gradient of diatrizoate forms within the density barrier. The PMNs band within this density gradient while the mononuclear cells remain at the sample/medium interface.
Each batch of Polymorphprep is checked on the level of endotoxins using a specific LAL test. Our goal is to produce batches with an endotoxin level lower or equal to 0.13 IU/ml.
The method is effective only with whole undiluted blood not with a leukocyte-rich fraction. The temperature is important to obtain optimal results, as changes in temperature effect the density and viscosity of the PolymorphprepTM solution. The temperature of the blood sample and the medium should be kept between 18-22 degree Celsius.
Analysis of the top and bottom bands from the PolymorphprepTM separation can be performed by a Coulter STKR Cell Analyser. The analyzer determines the number of cells in the sample (ordinate) as a function of cell volume (abscissa). Relative cell number is the number of cells of a particular volume expressed as a fraction of the total in each sample.
The cell band on top of the Polymorphprep contains only peripheral blood mononuclear cells (PBMCs). The cell band itself can be separated into lymphocytes (upper layer, 1) and monocytes (lower layer, 2). All of the PMNs are in the bottom cell band, which is enclosed of the Polymorphprep solution. Contamination of the PMN band by erythrocytes is between 2-6 % of the total cell number
References/Publications (1)
Downloads
Q & A's
Customer Reviews
Login