pOPE101 Expression Vector
Cassette vector for the expression of functional recombinant single-chain Fv antibody fragments in Escherichia coli (lacIq genotype (e.g. XL1-Blue).
Product description
| Quantity | 5 µg |
|---|---|
| Host | E. coli lacIq genotype (e.g. XL1-Blue) |
| Purification | Plasmid Purification Kit |
| Storage | -20°C |
| Intended use | Research use only |
| Concentration | 0.5 µg/µl in TE buffer |
| Purity | UV-Scan (220-320) with peak at 258 nm |
| Stability | Minimum 1 year when stored at -20°C |
| Vector | 3970 bp, AmpR |
| Cloning sites | MluI and NotI for light chain VL genes, NcoI and HindIII for heavy chain VH genes |
| Note | For plating we recommend to use LB Agar plates containing 100 mM Glucose and 100 µM Ampicillin |
Background
Introduction
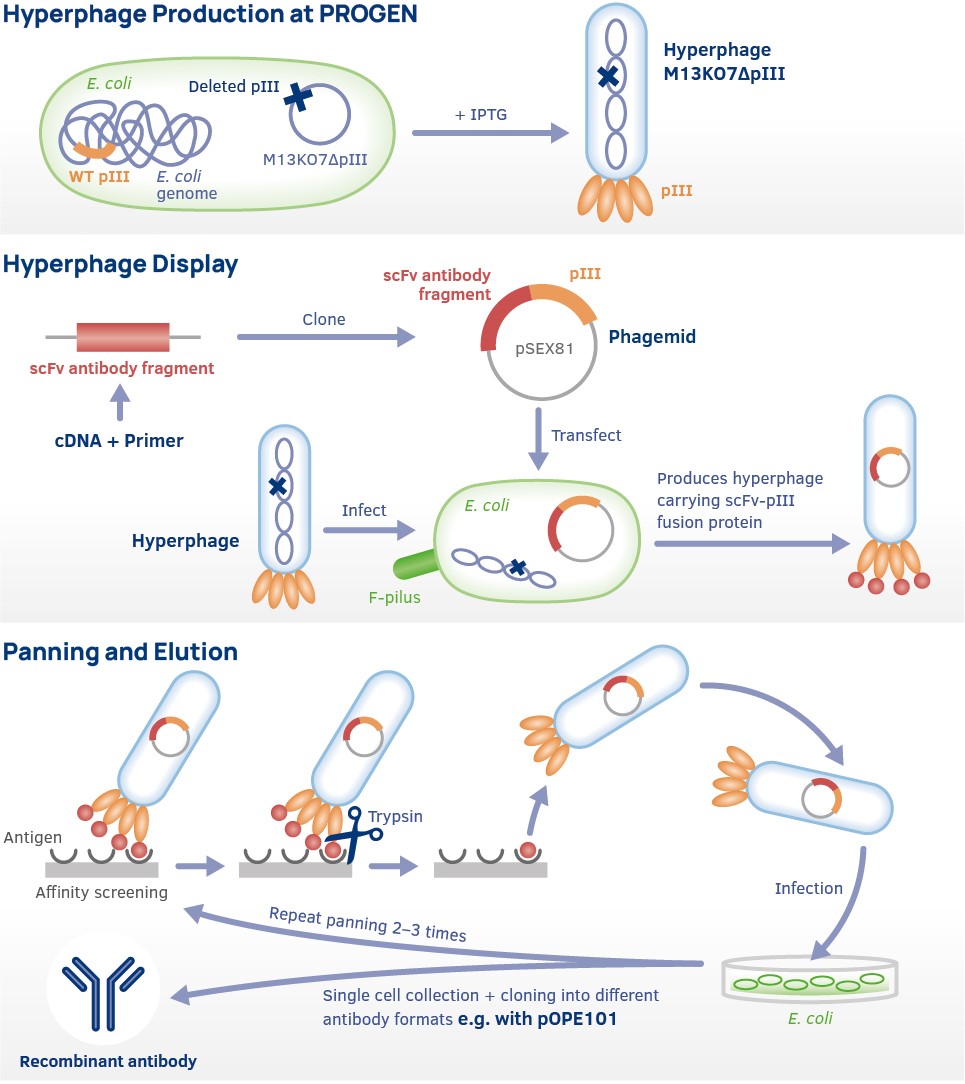
Complete immunoglobulin molecules cannot be produced in Escherichia coli, but within the past decade, recombinant antibody technologies have been widely used to express various recombinant single-chain Fv antibody fragments (scFv) of different specificity. ScFv antibody fragments have first been deposed as cytoplasmic inclusion bodies followed by refolding in vitro. Due to the general low folding efficiency, however, another strategy has been developed which better imitates the folding conditions of antibodies in eucaryotic cells. The secretion of the antibody fragments into the periplasmic space of E coli permits their production as soluble and functional proteins with correctly formed intramolecular disulfide bonds. The total yields are usually lower than those of inclusion body refolding, however, it is the method of choice for a rapid assessment of antigen binding activity and specificity.
PROGEN now offers the cassette vector pOPE101 (Genebank accession no. Y14585) for the expression of functional recombinant single-chain Fv antibody fragments in Escherichia coli. The vector was specifically designed for cloning of heavy and light chain Fv gene fragments isolated from hybridomas, individual B cell clones as well as antibody libraries by using PROGEN's PCR primer sets F2000 or F2010.
Vector Design
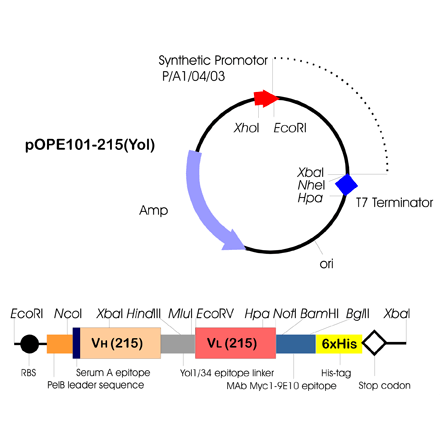
The cassette vector pOPE101 was designed for the convenient insertion of heavy and light chain variable domain coding regions and for production of functional monomeric single-chain Fv antibody fragments in E. coli (Schmiedl et al., 2000). The corresponding DNA fragments of human or mouse origin can be amplified by PCR using PROGEN's primer sets F2000 or F2010, respectively. The amplified gene fragments encoding the variable heavy and the variable light chain domain are cloned in-frame between a pelB-leader sequence for the secretion of the fusion protein into the periplasmic space, and a short region encoding tags to facilitate detection and purification. The VH and VL genes were joined by a DNA fragment coding for a flexible 18 amino acid residue linker containing the first six amino acids of the CH1 constant region domain and the hydrophilic pig brain apha-tubulin peptide sequence EEGEFSEAR. At the 3'-end of the VL domain coding region, a short DNA fragment codes for a peptide tag of the proto-oncogene product c-myc. It contains the linear epitope EEQKLISEEDL, which is recognised by the mouse monoclonal antibody mab Myc1- 9E10 (Evan et al., 1985). This is followed by six histidine residues, facilitating purification of the fusion protein by IMAC. The vector backbone further provides a strong synthetic promotor, the T7 terminator, the ColE1 origin of replication and an ampicillin resistance marker for selection.
In pOPE101 the recognition sites of the restriction endonucleases Nco I and Hind III allow the insertion of a VH gene fragment. For insertion of a VL gene fragment the sites of the restriction endonucleases Mlu I and Not I are recommended.
Material Required
Equipment for DNA cloning
Preparation of Reagents
The DNA should be diluted and transfered into E. coli cells as described in standard protocols. Use an overnight culture of a single clone to extract enough DNA for the following cloning procedures.
Cloning procedure
The immunoglobulin heavy and light chain Fv domain coding regions must be cloned in-frame into pOPE101 (see attached graphic). To clone a VH gene fragment the recognition sites of the restriction endonucleases Nco I and Hind III are recommended. The corresponding VL gene fragment should be introduced by using the recognition sites of the enzymes Mlu I and Not I. The cloning sites of the selected Fv gene fragments have to match the corresponding amino acid positions given by the vector. Gene fragments of genes derived from human or mouse origin by using PROGEN's PCR primer sets F2000 or F2010 can be directly cloned into pOPE101. After cloning, we recommend to check the sequence of the inserts to confirm that no mutation occured in the open reading frames. The vector is now ready-to-use for the expression of functional recombinant singlechain Fv antibody fragments in Escherichia coli.
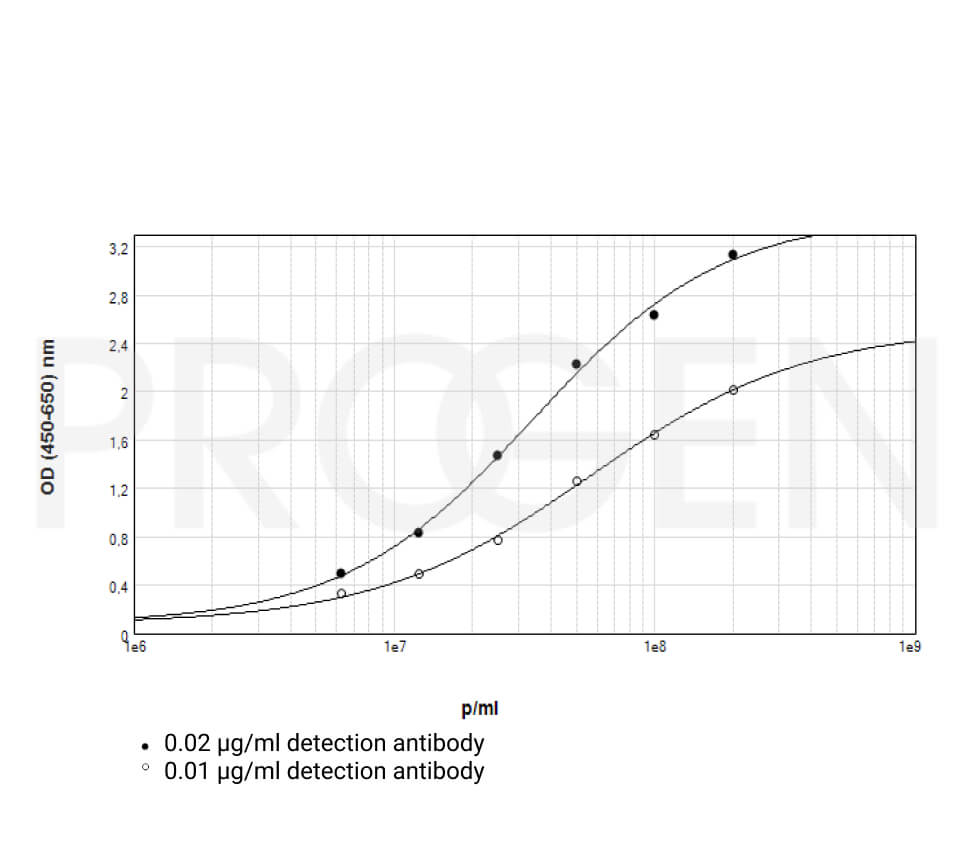
The expressed antibody fragments can be purified from periplasmic extracts as described in standard protocols (Schmiedl et al., 2000; Schmiedl and Duebel, 2001).
References/Publications (13)
Downloads
Q & A's
Customer Reviews
Login
FAQs
DH5alpha or TOP10 (without F episome) are lacIq negative and therefore the toxic proteins are expressed in these cells. When using lacIq negative cell lines with the pSEX81 vector you will receive only a few colonies and most often the plasmid is somehow mutated in these colonies.
We also recommend to include 100 mM Glucose to the culture medium to avoid possible mutations of the plasmid.
Only vectors coding for a fully functional PIII protein are suitable for the hyperphage system, for example pSEX81.
According to the literature pCANTAB vector is compatible with hyperphage, although with lower efficiency as for example pSEX81 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1866265/).