Key Features
- Highly effective density gradient media for the separation of full and empty AAV particles
- Designed for the purification of viruses (such as AAV)
- Useful to isolate different cell types and organelles
- Ready-made, sterile and endotoxin-tested solution of iodixanol
Product description
| Quantity | 1x250 ml |
|---|---|
| Intended use | Research use only |
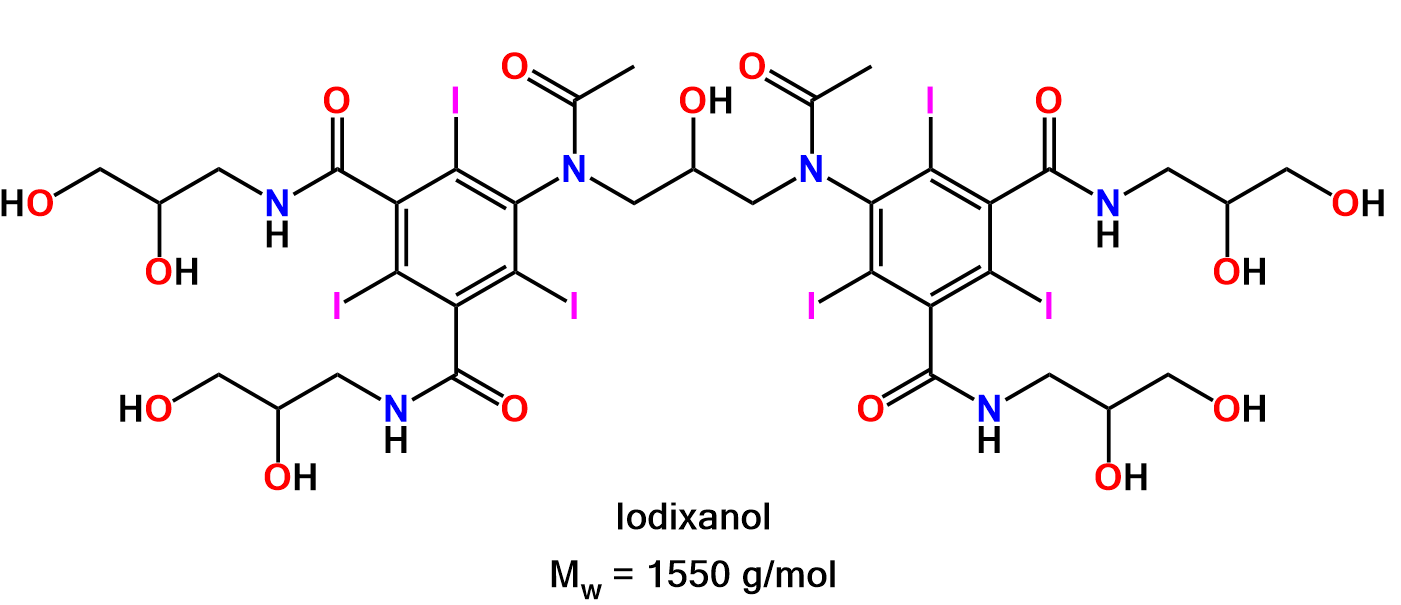
| Product description | OptiPrep™ is a ready made, sterile and endotoxin tested solution of Iodixanol, 5,5’-[(2-hydroxy-1-3 propanediyl)-bis(acetylamino)] bis [N,N’- bis(2,3 dihydroxypropyl- 2,4,6-triiodo-1, 3-benzenecarboxamide], designed for the in vitro isolation of biological particles. |
| Density | 1.320 +/- 0.001 g/ml |
| Composition | Iodixanol: 60% (w/v) in water |
| Osmolality | 170 ± 15 mOsm |
| Endotoxin | < 1.0 EU/ml |
| Stability & storage | OptiPrep™ is stable for 3 years provided the solution is kept sterile and protected from light. Prolonged exposure to direct sunlight leads to release of iodine from the molecule. This effect is negligible when working with these solutions on a day to day basis. OptiPrep™ should be stored between 4°C - 24°C. Before use invert the bottles several times to mix the contents. |
Background
OptiPrepTM is a sterile endotoxin tested solution of 60% iodixanol in water. Iodixanol was developed as an X-ray contrast medium and has therefore been subjected to rigorous clinical testing. Iodixanol is non-ionic, non-toxic to cells and metabolically inert. Iodixanol solutions can be made iso-osmotic at all useful densities. Iodixanol solutions have low viscosity and osmolality. Actual endotoxin levels in each batch are usually measured at ≤ 0.13 EU/ml.
Applications:
1. Purification of viral particles such as AAV
OptiPrepTM is the ideal solution for virus purification. Using OptiPrepTM gradients, the ratio of infectious particles to total particles is 100 times higher than a virus purified using CsCI gradients.
Benefits of OptiPrepTM for AAV purification:
Benefits of OptiPrepTM for AAV purification:
- Saves time – no need to remove iodixanol for most downstream processes
- Not destructive – causes little or no damage to viral particles
- Greater recovery rate – ten times greater than CsCI gradient alternatives
- Higher infectivity titer –no toxic effects
2. Isolation of full and empty AAV capsids
Decreasing empty capsids in your AAV preparation ensures product safety by minimizing the risk of unwanted immune responses and maximizing transduction efficiency of your AAV gene therapy product.
The key advantages of removing empty capsids:
- Reduce patient risk by minimizing unwanted immune responses
- Maximize your success rate – improved transduction efficiency allows you to use lower titers
- Be ready for clinical trials – focusing on increased safety ensures a smoother transfer
- Manufacture high quality products – by combining with your chromatography manufacturing process
3. Isolation of other biological particles
The high density of OptiPrepTM facilitates the fractionation of cells by flotation from a dense load zone through either a continuous or discontinuous gradient or through a simple density barrier.
The low viscosity of the isoosmotic gradient (OptiprepTM) provides a rapid and efficient separation as well as an improved resolution of the major cell organelles.
The low viscosity of the isoosmotic gradient (OptiprepTM) provides a rapid and efficient separation as well as an improved resolution of the major cell organelles.
Additionally, the isolation of the following is possible:
- Mammalian and non-mammalian cells
- Subcellular organelles
- Plasma membranes and domains
- Membrane vesicles and cytosol
- Organelles from non-mammalian sources
- Plasma lipoproteins
- Proteins and protein complexes
- Plasmid DNA
- Ribonucleoproteins
Protocols and references are available for the isolation of cells, macromolecules, subcellular organelles and membranes and virus purification.
For every batch produced a Certificate of Analysis showing the actual values of density and endotoxin levels is made available upon request. We also claim sterility according to Ph.Eur.
OptiPrepTM is manufactured, packed and released in compliance with GMP and ISO 13485.
Downloads
File
Category
Size
Filetype
Q & A's
There aren't any asked questions yet.
Customer Reviews
Login