Introducing Recombinant Adeno-associated Viruses (rAAVs)
Over the last decade, gene therapy has proven to be a powerful treatment option for patients with genetic diseases with adeno-associated viruses (AAVs) being among the most effective vehicles for delivering genetic material to a patients’ cells. Because they are nonpathogenic and have low cytotoxicity and low immunogenicity, AAVs are well suited for this purpose. But they are not used for gene therapy in their naturally existing state, rather, there are certain key changes that are made to improve their efficiency and enable them to better target specific tissues.
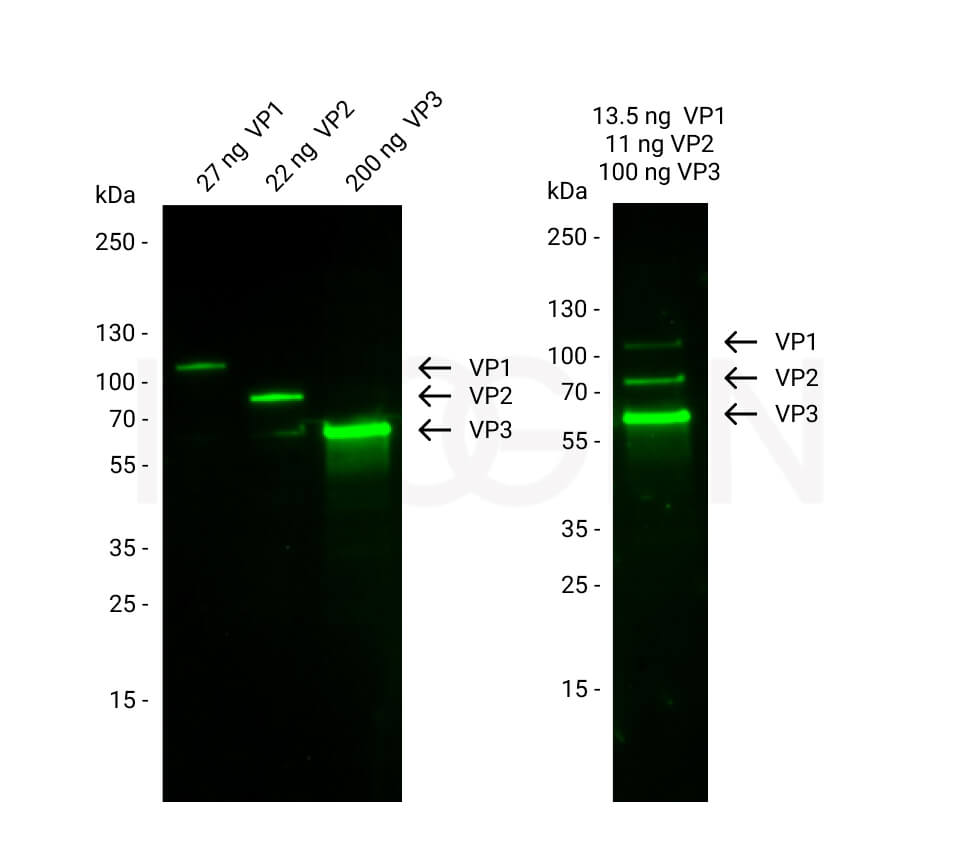
An AAV produced by rearranging DNA is known as a recombinant AAV (rAAV). Removing the viral DNA from an AAV and replacing it with the transgene of interest, which makes it possible to generate a gene therapy targeting a specific genetic disease is one way of producing an rAAV. While a wild type AAV consists of replication (rep) and capsid protein (cap) genes flanked by two inverted terminal repeats (ITRs), in an rAAV gene therapy product, the rep and cap genes are replaced by the transgene. Thereby, rAAVs are able to deliver the gene of interest and, in addition, lack the replication gene, so they cannot replicate, package the viral genome, or integrate into the host’s DNA, making them particularly safe gene therapy vehicles.
There is also a variety of modifications that can be made to AAV capsids in order to optimize their efficiency to transduce cells and specific target tissues. One way to do this is to combine different genomic elements of AAVs from different serotypes to create a more effective rAAV. A mosaic capsid can be created from a mixture of capsid subunits from different serotypes. This new capsid has beneficial properties from its various sources resulting in the rAAV entering a cell more efficiently, which, in turn, may result in an increase in transgene expression.
In addition, an AAV can be modified to improve its efficiency by replacing certain amino acids or domains, such as surface loops or residues, with those from capsid proteins from other serotypes to create a chimeric capsid. Other ways to enhance the effectiveness of AAVs includes inserting peptides into certain regions of the viral protein or by exchanging specific amino acids in the capsid protein through point mutations.
Despite the seeming complexity of these modifications, one notable benefit of rAAVs is that they are simple to produce in human cell lines. This fact, along with their proven effectiveness, makes rAAVs an excellent choice for gene therapy.
Interested in learning more about AAVs?