Performance Data
AAV Dip'n'Check Lateral Flow Tests can be used for a broad range of applications, ultimately helping to facilitate and accelerate AAV-based gene therapy.
Lets take a look at the variety of applications that the AAV Dip'n'Check Lateral Flow Tests can be used for.
- Comparing capsid titers of two unknown samples
- Titer estimation using a reference standard
- Spot landing in the optimal dynamic range
- Testing antibody binding to a variant AAV
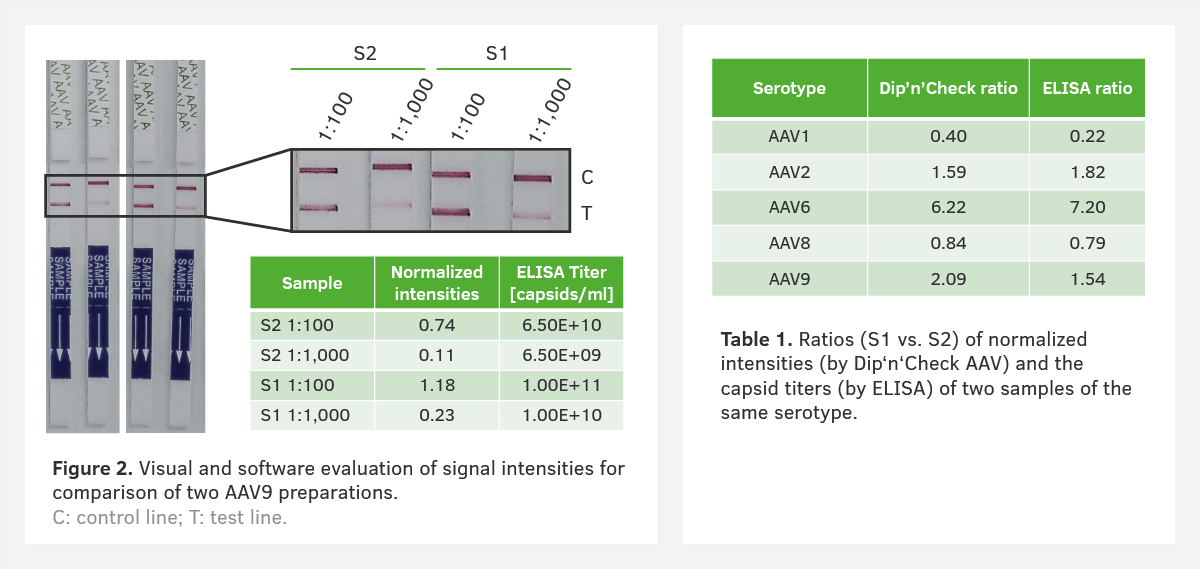
1. Comparing capsid titers of two unknown samples
When comparing two AAV9 preparations we could see a higher intensity for sample 1 (S1). This indicates that there is a higher capsid titer compared to sample 2 (S2). We were able to confirm this result using image analysis and our AAV ELISA (Figure 2). We used several serotypes to compare the AAV Dip'n'Check Tests against corresponding AAV ELISA data and the results showed a strong correlation between the two methods (Table 1).
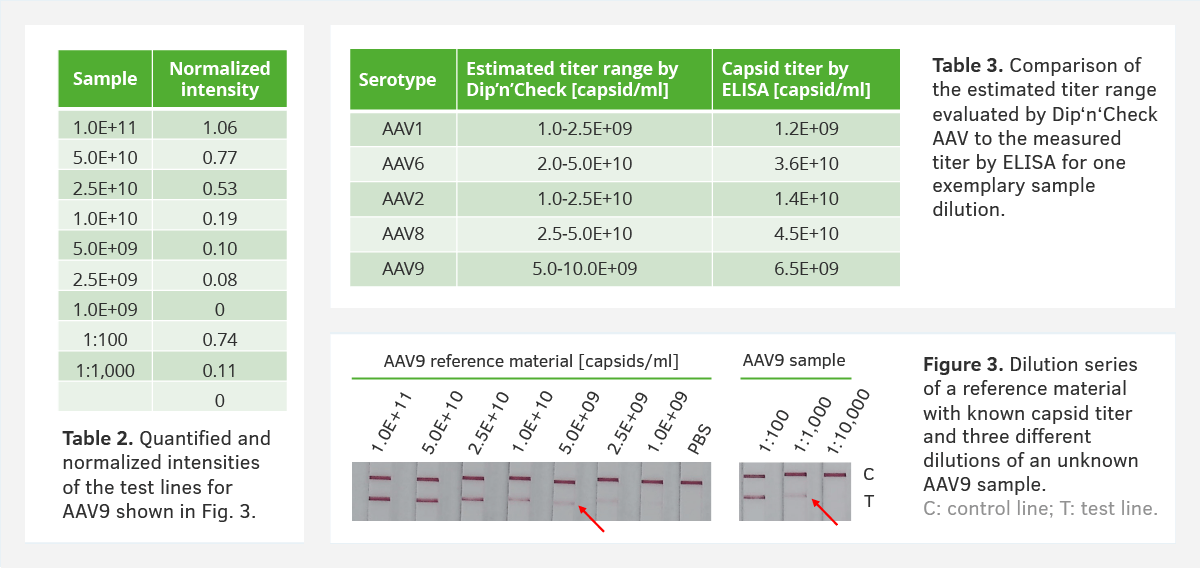
2. Titer estimation using a reference standard
AAV Dip’n’Check tests can be used as a titer estimation for AAV preparations, which you can then compare both visually and digitally with an AAV reference material (Figure 3 & Table 2). In Figure 3 you can see the AAV9 sample (1:1,000) has an estimated titer of approx. 5.0E+09 capsids/ml (see red arrows). We were able to confirm this data across different serotypes using the corresponding AAV ELISA titers (Table 3).

3. Spot landing in the optimal dynamic range
Most accurate capsid titer determination methods, for example the AAV ELISA, have a specific dynamic range. It can be challenging to find the correct dilution of unknown samples. But, by using the AAV Dip'n'Check tests to determine the titer of an unknown sample, you are able to hit the optimal dynamic range in the first attempt. In Figure 4, the 1:1,000 dilution of the AAV6 sample shows a similar intensity to the reference material, indicating that the undiluted sample has a capsid titer of approx. 5.0E+12 capsids/ml. Therefore, a dilution range between 1:25,000 to 1:100,000 allows you to hit the optimal dynamic range to determine the accurate titer required for the AAV ELISA.
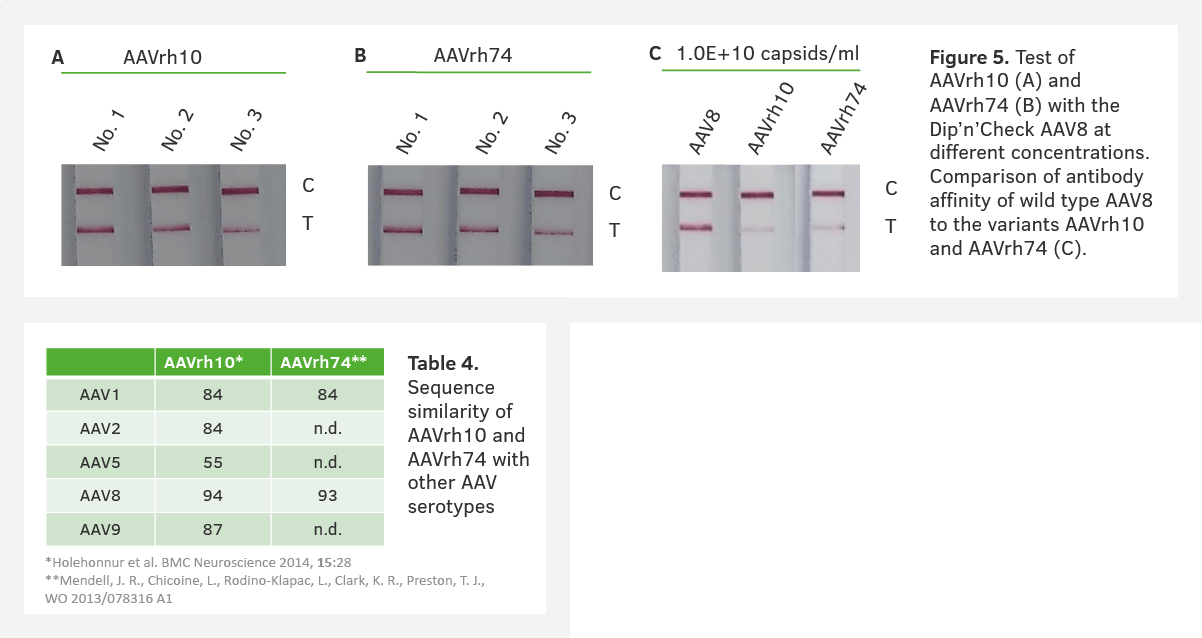
4. Testing antibody binding to a variant AAV
Before using antibody-based methods (e.g. ELISA) for AAV capsid variants, you need to confirm the binding of AAV antibodies to the engineered capsid. Pre-testing the antibody binding using AAV Dip’n’Check Tests allows you to determine which AAV ELISA is most suitable. The binding capabilities of AAVrh10 and AAVrh74 to the ADK8 were confirmed by testing different dilutions of both serotypes with the AAV8 Dip’n’Check Tests (Figure 5, A & B). In contrast, the binding affinity was analyzed by testing similar dilutions of AAV8, AAVrh10 and AAVrh74 and comparing the signal intensities (Figure 5, C). The results from the AAV Dip’n’Check Tests show the AAV8 ELISA is suitable for use with AAVrh10 and AAVrh74. However, it also indicates that it is necessary to adapt the AAV ELISA due to the lower affinity of the antibody to the capsid variants.
Potential Applications for AAV Dip'n'Check
AAV is the most commonly used viral vector for the delivery of therapeutic transgenes in academia and industry. However, the gene therapy community is facing major challenges, such as the high demand for effective and reliable analytical AAV tools for gene therapy manufacturing and develompent
This poster shows the potential applications of AAV lateral flow assays and how they can help accelarate your next AAV gene-therapy based project.
1. Comparing the capsid titer of two unknown samples
2. Estimating the titer using a reference standards
3. Spot landing in the optimal dynamic range
4. Testing antibody binding to variatn AAV serotypes

AAV Dip'n'Check Tests
A quick and easy in-process control for AAV capsid purification processes or analytical pre-testing

AAV Titration ELISA
ELISAs for quantification and detection of
full and empty capsids.

AAV Standards
Reliable reference materials to ensure the validity of your results and comparability across assays.


